Research
Our research seeks to address fundamental questions about how sarcoma cells adapt and evolve in response to their environment and treatment.
We invite you to consider joining our team.

Cell State Plasticity in Rhabdomyosarcoma Progression
One of the main focuses of the Hebron lab is a pediatric soft tissue sarcoma called rhabdomyosarcoma (RMS), which resembles dedifferentiated skeletal muscle cells. As opposed to many adult cancers, pediatric cancers are more commonly associated with defects in developmental signaling programs than environmental exposures.
Because of altered developmental signaling, pediatric sarcoma cells often exhibit greater control over their differentiation than healthy cells, a phenomenon called cell-state plasticity. Cancer cells use this ability to evade therapies (resistance), spread to other areas of the body (metastasis), and regrow after treatment (relapse). We are working to understand the role of the proteins YAP1 and TAZ in rhabdomyosarcoma plasticity to identify new therapeutic strategies for patients with resistant, relapsed, or metastatic RMS.
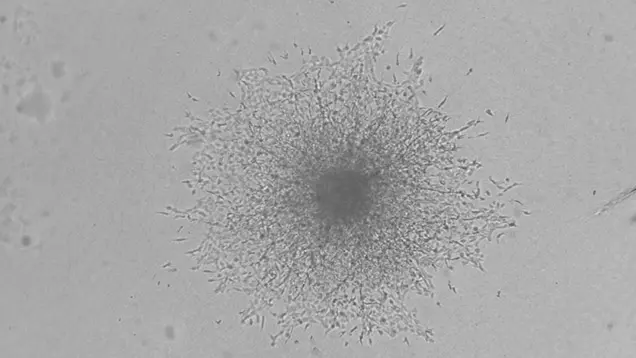
Rhabdomyosarcoma Invasion and Extracellular Matrix Reorganization
Tumor cells exist within a complex community of normal, altered, and malignant cells within the body, referred to as the tumor microenvironment (TME). The TME comprises many elements, including cancer cells, immune cells, blood and lymphatic vessels, nerves, fibroblasts, and structural components such as the extracellular matrix (ECM).
Crosstalk between these elements plays a vital role in how the cancer cells survive, respond to therapy, and spread. In this project, we will determine how tumor cells interact with, remodel, and exploit the tumor microenvironment, especially the ECM, to evade therapy and spread.
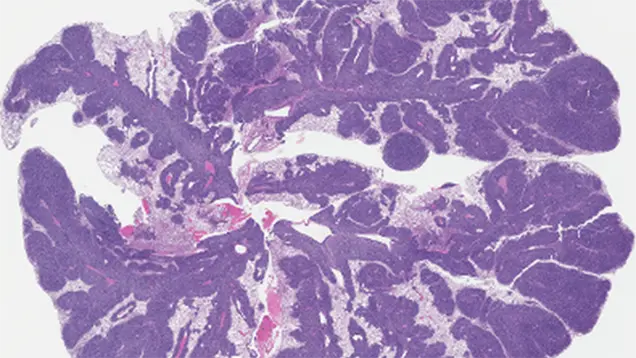
Primary Site-Specific Regulation of Sarcoma Metastasis
For sarcoma patients, some primary tumor locations are associated with a higher incidence of metastasis and poorer outcomes. However, the biology (and potential for therapeutic intervention) driving this prognostic factor is not well defined. Our preliminary data suggest a prominent primary site-specific role for the tumor microenvironment (TME) in sarcoma progression.
In this project, we will use innovative models to define these site-specific effects of the TME on invasion, metastasis, and tumor plasticity, bringing the field closer to understanding the mechanisms of sarcoma metastasis and identifying metastasis-targeting agents.

